Postmarket Surveillance of Medical Devices: A Comparison of Strategies in the US, EU, Japan, and China | PLOS Medicine
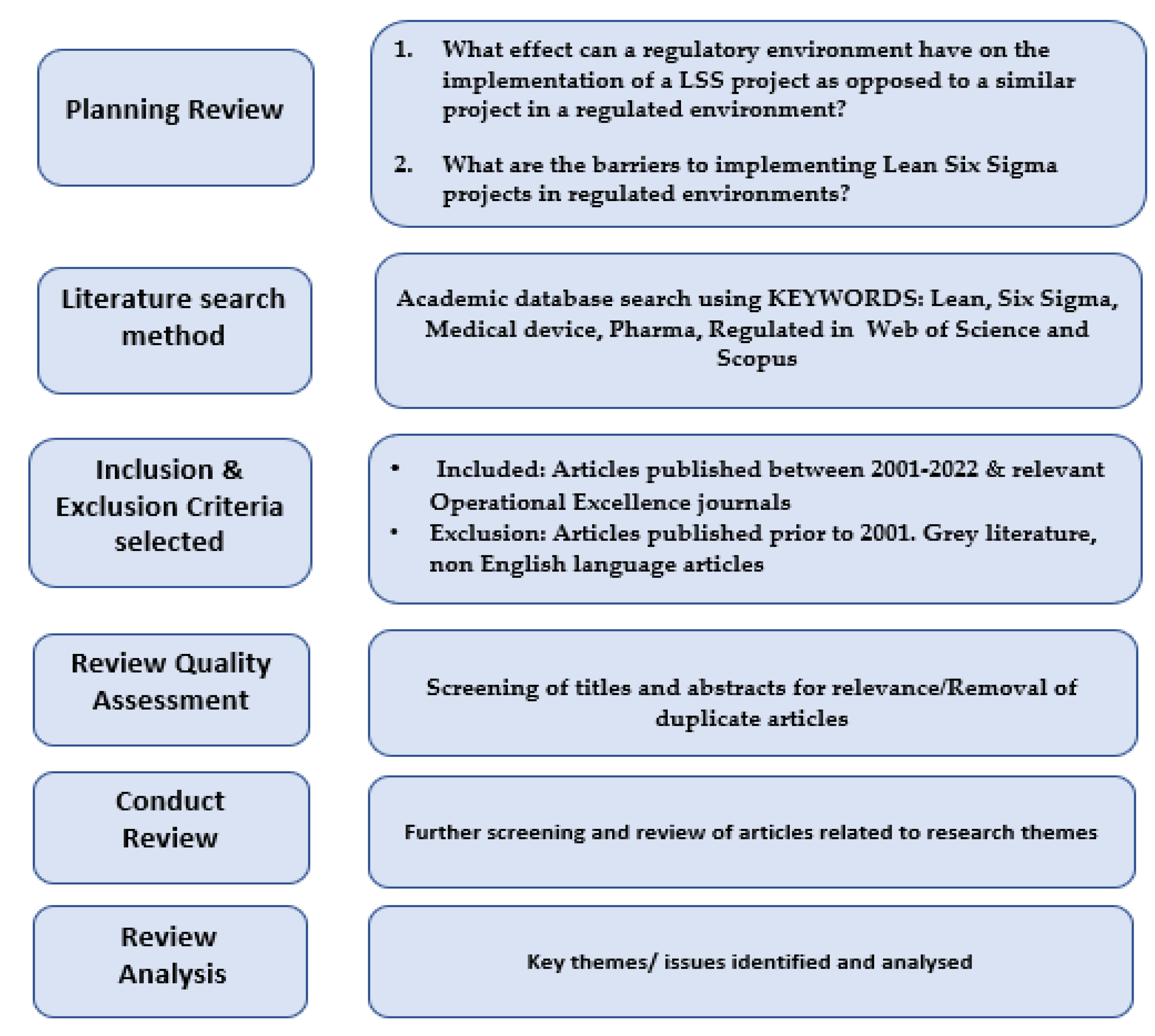
Processes | Free Full-Text | The Effect of Medical Device Regulations on Deploying a Lean Six Sigma Project

The Food and Drug Administration's (FDA's) 510(k) Process: A Systematic Review of 1000 Cases - The American Journal of Medicine

New Medical Device and Therapeutic Approvals in Otolaryngology: State of the Art Review of 2021 - Alexander M. Choi, Michael J. Brenner, Daniel Gorelik, Isaac D. Erbele, Matthew G. Crowson, Prajoy Kadkade,

In vitro stability of biosimilar insulin aspart SAR341402 in the Medtronic MiniMed insulin pumps - Journal of Pharmaceutical Sciences
Hearing Aids and Devices Including Wearable, Bone Anchored and Semi-Implantable – Individual Exchange Medical Policy
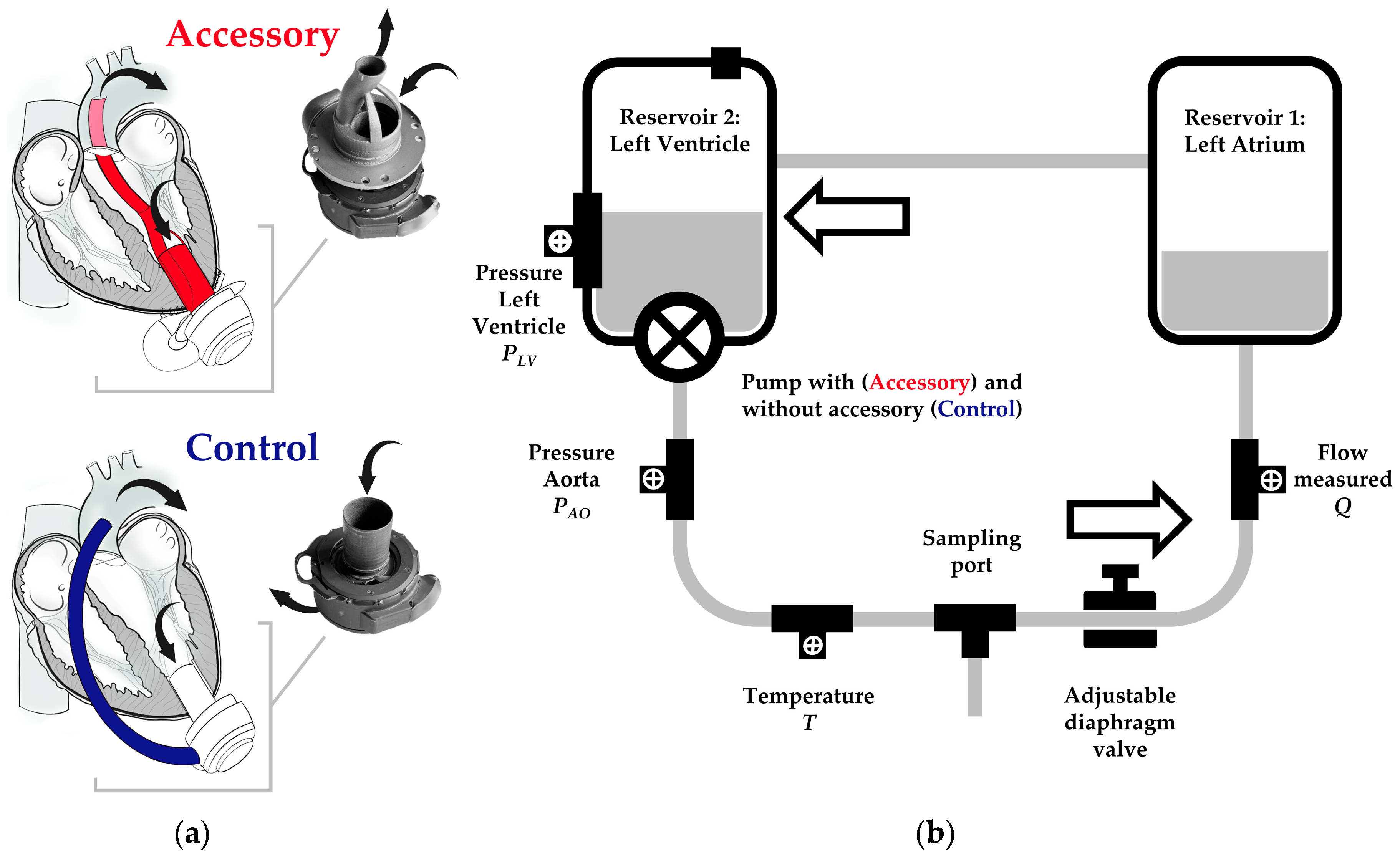
Bioengineering | Free Full-Text | Impact of an Accessory for Left Ventricular Assist Devices on Device Flow and Pressure Head In Vitro

Long-Term Clinical Effectiveness of a Drug-Coated Balloon for the Treatment of Femoropopliteal Lesions | Circulation: Cardiovascular Interventions

American Association of Clinical Endocrinology Clinical Practice Guideline: The Use of Advanced Technology in the Management of Persons With Diabetes Mellitus - Endocrine Practice

Study Design and Data Analysis of Artificial Pancreas Device Systems with Closed-Loop Glucose-Sensing Insulin Delivery

Clinical Evidence Supporting FDA Clearance of First-of-a-Kind Therapeutic Devices via the De Novo Pathway Between 2011 and 2019 | medRxiv